I learned a lot from the experiment and came out of it with at least one bit of useful information. Will I try to restrict salt in my diet? Na (sorry, couldn't resist). I don't think salt restriction can work for me. From now on I will ensure that I get sufficient salt on a daily basis.
Executive Summary
Cutting to the chase, these are the main points I learned over the course of the experiment, roughly sorted from most to least interesting.
1. Salt restriction caused impaired thermoregulation. In hot weather, my cardiovascular system was not able to sufficiently lower my body temperature. This resulted in an elevated heart rate and hypethermia (up to 101.5 degrees in one instance). This can be dangerous, so be careful if you try this at home.
2. No clinically meaningful change in blood pressure. Systolic pressure was unchanged, though salt loading may have caused a small rise in diastolic pressure. This does not rule out long term negative effects from chronic salt loading (see discussion below), but it does show that, as previously discussed, my kidneys seem to basically work and can regulate my blood pressure through the maintenance of fluid and electrolyte balance in response to changes in my sodium intake.
3. Possible susceptibility to skin infections. Three days into the salt restriction phase, I came down with what was probably a staph infection in my right eyelid. This responded to antibiotics but it came back once I went off them. Since adding back salt I have had no problems with skin infections and no more antibiotics.
6. Bodyweight changes. I experienced substantial changes in body fluid levels (e.g. 6 pound weight gain within two hours of the transition from the salt restriction to the salt loading phase).
Conclusion: A low carb paleo diet must include added salt (for me). Can others do without? Perhaps, and some scientists such as Loren Cordain and Tim Noakes (e.g. this podcast episode 18 at 1:03:50) seem to think they can. Skip ahead to read my further musings on this question.
Study Design
The experiment was conducted in three phases. First, I did a one week lead-in phase (phase I) where I made no changes to diet or salt consumption. The purpose of phase I was to establish a blood pressure baseline through daily morning measurements (see Measurement Methods below).
This was followed by a three-week sodium restricted phase (phase II) during which I did not add any salt to my food. In addition, during phase II only, I avoided naturally salty foods such as shellfish. My sodium intake during phase II was limited to the sodium in the foods I was eating. Note however that there were one or two restaurant meals per week during this time where I was not able to strictly control for added salt. Sodium consumption on phase II was estimated to be between 800mg and 1000mg per day. phase II was originally scheduled for two weeks, but was extended due to the aforementioned infection and antibiotic use.
Finally, phase III was a salt-loading phase during which I added an additional 5 grams of sodium to my diet, for a total of nearly 6 grams of sodium per day including the sodium naturally occurring in my food. The supplemental salt during phase III consisted of hand harvested French Celtic sea salt (Eden Foods, Inc.) and was measured daily on an AMW-1000 digital scale. Because the Eden French Celtic sea salt is approximately 1/3 sodium by weight according to the label, the 5 grams of supplemental sodium per day was provided by approximately 15 grams of sea salt. Note that different varieties of salt will contain different percentages of sodium by weight. Sea salts vary significantly due to variations in residual water content (not, as commonly assumed, by the presence of other minerals). Please consult the label or a friendly analytical chemist for guidance.
The diet throughout this experiment consisted of meat, fish, eggs, coconut oil, butter, and non-starchy vegetables. In addition, I typically consumed a banana, an ounce (28g) of almonds and a bit of dark chocolate each day. Potassium intake was fairly consistent at around 4 g/day. Table 1 shows a typical day's macronutrient intake. Given the macronutrient ratio, I believe it is likely that the diet was ketogenic.
Table 1. Approximate daily macronutrient intake.
| Macronutrient |
grams
| calories | percent (calories) |
| Carbohydrate |
50
| 200 | 6.6% |
| Protein | 155 | 620 | 20.5% |
| Fat | 245 | 2205 | 72.9% |
| Total | 3025 |
100%
|
Measurement Methods
Blood pressure was measured daily first thing each morning while seated, with the cuff of an Omron HEM-711 placed on the left upper arm over the brachial artery. I followed guidelines described by Agena et al (see Chart 2 of the linked paper). Each day's blood pressure value was determined by averaging the first three measurements taken that morning.
My first measurement of the day was typically higher than the average of the second and third measurements (systolic: +5, diastolic: +4, average over all three phases). This is referred to as the "alarm reaction" and is related to the more commonly known "white coat syndrome", where the presence of a doctor elicits a stress response and therefore an innacurately high blood pressure reading. My alarm reaction seems to be due to the fact that I get slightly stressed out about seeing what my blood pressure is, even when I measure it myself. Therefore I experience a slight rise in blood pressure while waiting to see the first reading each day. I kept all three readings for this experiment. My "true" normal blood pressure is on average slightly lower than these results which include the first "alarm" reading.
Results
I summarized my qualitative findings in the executive summary above. If you skipped that because you are not an executive, you can go back and read it now. Below are graphs showing my blood pressure and bodyweight during the three phases.
 |
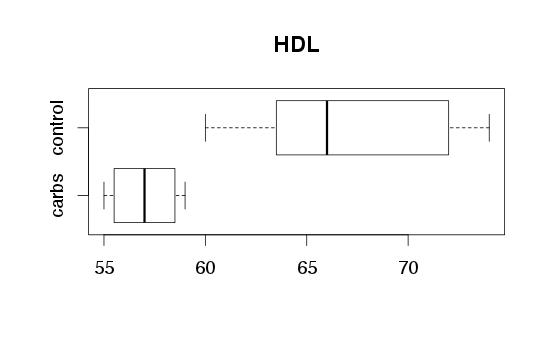
| Figure 2. No change in systolic blood pressure. |
 |
| Figure 3: Bodyweight. |
Figure 3 shows my daily bodyweight, measured each morning before consumption of any food or fluids. Note that my previous health goal (the 415 deadlift) involved an intentional increase in bodyweight and therefore significant excess calorie consumption. My current diet is lower in calories and Figure 3 therefore should show a long term downward trend in bodyweight.
Salt restriction clearly resulted in a rapid decrease in bodyweight over the first few days of phase II. There appears to be a stabilization towards the end of the salt restricted phase. The salt loading in phase III produced a very large initial weight gain, followed again by stabilization around the same level seen at the end of the salt restriction phase. As salt is primarily stored in bones and extracellular fluids, an increase in salt would be expected to correspond to an increase in extracellular fluid (since the body's bone mass should change slowly). The bodyweight changes shown in Figure 3 therefore reflect changes in extracellular fluid levels. While salt loading at the levels used in phase III produced a large acute change in body fluids, this was restored to normal over approximately 5 days.
Since my extracellular fluid volume was evidently restored within 5 days, it is not surprising that salt loading had no significant effect on my blood pressure. What is somewhat surprising was that there was no evidence of a temporary increase in blood pressure during the few days in which my extracellular fluid volume was in fact elevated. This suggests that there is an additional regulatory element working to restore blood pressure homeostasis at a shorter time scale than the dominant kidney-fluid mechanism previously discussed on the blog here.
Thanks to Mako Hill for guidance with ggplot2, without which these plots would look less nice.
Discussion
This experiment demonstrated to me that a low carb paleo diet with no added salt is potentially dangerous for me. Impaired thermoregulation is a big deal and would have been a life-threatening issue if I had to hunt for my food in a hot climate. Not only was my body temperature elevated in warm weather, but my pulse was elevated as well, suggesting my cardiovascular system was unable to restore my body temperature to normal. I'm clearly not salt sensitive, and I do not function well with a low salt diet. However, genetic studies suggest the ancestral human genotype is associated with high levels of salt sensitivity and ability to function with very low sodium intakes. How did humans evolve these traits? And why don't I seem to have them?
A Faustian Kidney Bargain
Susumo Watanabe has proposed in interesting hypothesis about the evolution of sodium metabolism in hominids. The theory is laid out in a 2002 paper called "Uric Acid, Hominid Evolution, and the Pathogenesis of Salit-Sensitivity," published in the journal Hypertension. It goes something like this. At some point during the evolution of our common ancestor with gorillas and chimpanzees, a series of mutations inactivated the gene for urate oxidase, an enzyme that breaks down uric acid. As a consequence, we have much higher blood levels of uric acid than other mammals. These mutations seem to have occurred between 24 and 8 million years ago, during the miocene, when our ancestors were believed to be subsisting primarily on fruits and leaves. This diet would have been exceptionally low in sodium. Since there is evidence of multiple independent mutations in this gene in multiple primate lineages, it is thought that mutations deactivating urate oxidase were strongly selected.
In rats, uric acid raises blood pressure acutely, but also causes renal vascular disease via renin/angiotensin systems. This over time makes the rats more salt sensitive. If there is very little salt available, salt sensitivity can be a good thing. Watanabe argues that, where salt is scarce, high uric acid is beneficial (via multiple pathways) for preventing blood pressure from going too low.
In addition to causing kidney disease, high uric acid causes other problems, like gout, and is associated with heart disease. So this looks like an engineering tradeoff with a number of downsides, but some benefits in the context of a miocene diet that was even lower in sodium than the lowest current estimates for paleolithic diets. The organism with this adaptation is supposed to partially destroy its kidneys on purpose in order to maintain sufficiently high blood pressure. This miocene environment is long gone. However, it is much easier to break a gene than to put it back together. Our urate oxidase gene has been broken more than once and it would take quite a long time to fix it.
It's kind of a crazy theory. I'm not sure I believe it but it is interesting to think about.
Some Hypotheses
During this experiment, I was eating almost exclusively meat, fish (often with bones), eggs and vegetables, plus added calories from butter, coconut oil and olive oil. The diet was grain, legume and dairy free and, as mentioned, possibly ketogenic. This would be considered by many online diet and health personalities to be a good low carb paleo diet, even though of course processed fats like butter and coconut oil are not Paleolithic foods.
So I want to discuss a few possible ways to resolve the apparent impossibility of eating this way without added salt.
Hypothesis 1: Low Carb, Low Crab, or Low Salt: choose any two
I have been eating a low carb diet, and my experiment suggests that, in that context, low salt is not a good idea. It is possible that a healthy human diet can be either low in carbohydrates or low in salt, but not both.
A great deal of evidence suggests that ketosis was not the norm for our paleolithic ancestors (see e.g. Kuipers et. al. 2012 for a thorough review of paleolithic diet research). In fact it would have been quite a struggle for me to eat this sort of macronutrient ratio without modern refined fats such as butter and coconut oil. Or ready access to marine mammal blubber (but then again the Inuit are not my paleolithic ancestors).
In contrast to the online paleo diet scene, most low carb diet advocates seem to line up behind the recommendation for ample supplementary salt. My result accord with that clinical experience. Low carbohydrate diets are usually said to have a diuretic effect in this community, at least in the initial stages (e.g. M.R. Eades, Jenny Ruhl). It is possible that my problems were caused by the interaction between diet-induced ketosis and salt restriction, and I would have done just fine without salt if I had some more carbohydrates. This hypothesis would be straightforward to test.
In order to keep my sodium intake sufficiently low during the salt restriction phase, I had to remove shellfish such as oysters and mussels from my diet. Crab is also salty and makes for a handy pun. It seems likely that daily shellfish consumption would have pushed my sodium intake into the healthy range. While shellfish does not get much attention these days in the paleo club, there is ample support (again see Kuipers et. al.) that it was an important contributor to actual paleolithic nutrition.
Hypothesis 2: Humans must drink blood. Or eat salt.
File this one in the "teen paranormal romance" department. This hypothesis states that the ancestral human diet was not as low in salt as commonly assumed.
Sodium is the body's primary extracellular cation, and most of it is located in the blood and other extracellular fluids. A pint of blood contains about 1.6 grams of sodium (see, e.g., these livestock reference ranges for blood sodium). That much blood per day should have been more than enough to push me into the healthy range of sodium consumption. On the other hand, salt depletion set in pretty quickly for me (probably 3-4 days), so this hypothesis assumes that fresh blood was consistently available to inland populations that did not have ready access to shellfish or sea water.
I find this hypothesis intriguing because of the fact that my putative ancestors were commanded not to drink blood (Genesis 9:4, Leviticus 17:13, Deuteronomy 12:15-16), and that salt is used in this tradition specifically to remove blood from meat before it is eaten. Presumably blood drinking was outlawed because it was thought to spread diseases and not because of tacky pop-culture connotations. Were my ancestors salting their meat not just for its preservative qualities, but also to make up for the reduction in sodium intake due to their prohibition on drinking blood?
Hypothesis 3: I'm Not (Genetically) a Paleolithic Human
Some say the human genome has hardly changed in the past 10,000 years. However, the hard evidence points to a number of significant evolutionary changes since the advent of agriculture, the classic example being lactase persistance (see Cochran and Harpending 2009 for a thorough argument on the rapidity of recent human evolution). Genes associated with hypertension and salt sensitivity are also apparently under strong evolutionary pressure. Alan Weder discusses this in an article published in 2007 in the journal Hypertension about evolution and hypertension. It is worth reading as an example of excellent science writing.
My experiment clearly demonstrates that I am not salt sensitive. This is not surprising given my European ancestry. As discussed by Weber, the genetics of salt resistance seem to correlate with adaptations to colder climates. It seems possible that in the course of such adaptation, my ancestors lost the ability to function optimally on a low salt diet.
Is a High Salt Diet Safe?
It is possible that, as much of mainstream medicine believes, a high salt diet actually is unhealthy over the long term. There is nothing in this experiment that contradicts that belief. Just because I am resistant to the short term blood pressure effects of salt loading, that does not mean I am immune to whatever long term negative effects a high salt diet may have. While epidemiological studies have their problems, it seems unwise to discount their findings altogether.
Edward Frohlich has argued that, notwithstanding the fact that most people's blood pressure does not respond to acute increases in sodium intake, sodium is nevertheless responsible long-term for increases in blood pressure. He argues that excess salt causes kidney damage over time (as with uric acid this is mediated by renin/angiotensin systems), resulting long-term in an increase in blood pressure. While much of this research is based on studies done on rats (including those of the "spontaneously hypertensive" variety), this line of thought is worth looking into and I will continue to do so.
Further Research is Needed
Studies always end with the statement that further research, and therefore research funding, is required. Well, I don't need funding but I will go a little further than a call for further research. Here are some ideas for areas of study and self experimentation.
- High carb low salt diet -- measure bodyweight and thermoregulation (e.g. via controlled hot baths or showers) over 5 day intervention period.
- Anthropological studies of salt and carbohydrate consumption among hunter-gatherers. Including seasonal variation. Genetics would be expected to play a significant role as there is ample evidence for recent evolution of salt sensitivity.
- Theories and evidence for salt use among paleolithic people. Support for consistent blood and/or shellfish consumption? Evidence for use of natural salt deposits?
- Sodium consumption and skin infections. Is salty sweat bacteriostatic within in sweat glands? Is low sodium consumption associated with skin infections? How salt sensitive is staph aureus? How about p. acnes? Have comensal skin bacteria evolved higher or lower levels of salt sensitivity? Are there differences between human populations (e.g. African vs. European skin flora)?
- Evidence for genetic selection of salt sensitivity in neanderthals. Is it possible I inherited some of my salt-related genetics from my neanderthal ancestors? If salt resistance is associated with adaptation to cold environments (see Weber), neanderthals would be expected to show these adaptations, and if they conferred a selective advantage, they should have been propagated if passed to human populations.